With the scarcity that has hit the fuel markets, there are new innovations that scientists are coming up with as alternative fuel sources. To that effect, hydrogen chemical is currently being used as fuel in addition to producing electricity. It is made up of a single proton and the most popular and commonly used gas in the world.
It has the ability to carry energy from one place to another thus its conductivity is good. Furthermore, hydrogen is mostly used in vehicles thus it is primarily made within the fuel cell. The process of making hydrogen fuel is very simple as it involves separation of water, natural gas molecules and biomass from a hydrogen atom through two main ways that is simple electrolysis and steam reforming.
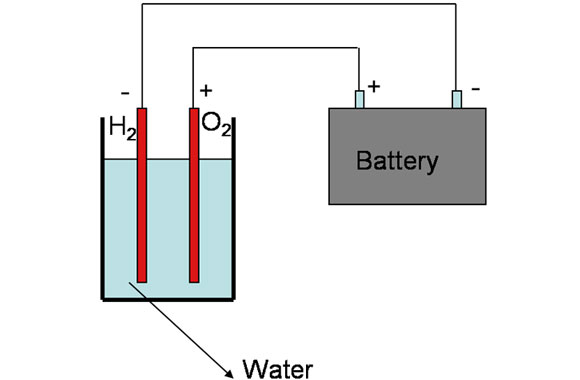
Electrolysis
Electrolysis remains to be the easiest of them all as it only involves electric currents that are run through water leading to the splitting of hydrogen atoms. The resultant gas is then released from water by the means of bubbles and separated through density. The main undoing of this method is that very little amounts of fuel are produced thus cannot be relied upon where large amounts are needed.
There is also the Sodium and water trick method that can be applied to come up with hydrogen fuel even though it is not very popular in the modern world. When Sodium is mixed with water they react making oxygen to separate with the molecules found in water with the resultant gas being hydrogen. This method requires a lot of heat to complete thus is well suited when there is need for mass production of hydrogen.
Vaporization
Vaporization is yet another of the industrial method of making hydrogen fuel. This involves the heating of fossil fuels to separate the hydrocarbons that contain carbon and hydrogen. When they fly apart then the gas is produced and after being collected the waste products is discarded. This method is not that efficient as it demands large amounts of heat even though its popularity is due to the fact that fossils themselves are popular.
The actual steps of making hydrogen fuel are very simple and easy for anyone to understand. The hydrogen stored in the fuel cells is run on one side while oxygen runs on the opposite. It is then split into negative and positive protons which are responsible for the production of the fuel. An electrical current is made when the negative protons move through a circuit which makes the vehicle or any other machine using the fuel to start.
The by-products
There is usually water produced as a by-product of the whole process when the positive and negative protons come together once more. The fact that hydrogen is a renewable source of energy makes it more efficient source of fuel as it does not pollute the environment in any way and can be produced through a variety of raw materials. Furthermore, hydrogen is found on earth as a component of several other elements which may include hydrocarbons that are often in form of natural gas as well as water.