Here at cleangreenenergyzone.com our aim is to provide reliable and up-to-date information in regards to green and renewable energy. This is a subject that has become increasingly important in recent years and we believe that by providing information to our visitors we will be giving them power to make a difference. We want our readers to have a much better understanding of renewable energy and to appreciate how they too can make a difference - all of us can play a part in this.
There is a lot of talk in the world press about the need to reduce our dependence on oil. This is something that we here at cleangreenenergyzone.com fully agree with and throughout this website you find suggestions about how this will be possible. Our aim is not to preach but only to provide practical information that will help us individually and by doing so create a greener world.
What is Green (Renewable) Energy?
Although we often hear people talking about green/renewable energy there are probably many of us who are still a bit confused as to what this means exactly. A simple way of describing what we are referring to here would be to say that this is a type of energy that we can generate using natural sources - the great thing about these natural sources such as solar power is that we don't have to worry about them running out any time soon.
Types of Renewable Energy
You will probably already have heard of some of the more popular ways of creating renewable energy. They are often talked about in the media and you may even have seen examples of these in actual use. Some of these renewable energy options have been in use for a number of years already while others are less well known. On this website we will focus mostly on the following types of renewable energy;
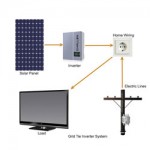
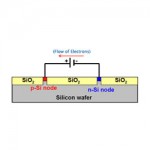
- Solar Power and Panels. This type of renewable energy is obtained from the sun by taking advantage of the light and heat that comes from it. There are already many examples of people taking advantage of this possibility. The possibilities available with solar power are increasing all the time.
- Wind Power and Wind Turbines. This takes advantage of the power of the wind to create energy. Wind turbines can provide energy for use in a building directly or it can be added to the national grid in a country as an additional energy source. In countries such as the UK there is great potential for this type of renewable energy.
- Biomass. This involves creating energy from organic material created by plants and animals. This is an interesting option for creating energy because it involves using things like manure and certain types of garbage as well as wood and crops. This is considered a renewable source because anything we use can be replaced.

- Hydrogen Energy - Hydrogen is something that we have no shortage of in the universe - it is everywhere. It also contains the highest amount of energy content that we are aware of. Hydrogen has already been used to power vehicles and to create fuel cells.
- Hydropower. This is another exciting way to create electricity and it involves using mechanical energy created by the water. In places like the United States hydropower is an important source of their energy requirements (around 6%). It is likely that the use of hydropower will increase in the future.
Advantage of Using Green Energy Resources
There are many advantages of green energy sources and we will list the most commonly spoken of here.
- Once a green energy resource is created it is relatively cheap to maintain and can provide energy for a long time.
- The price of many of the fuels we use today is likely to increase as they become scarcer - this is simple supply and demand. Green energy offers away out of this vicious circle of increasing expense.
- We should never need to worry about running out of renewable energy sources. This means that we can feel more confident about the future.
- Green energy resources can help humans reduce the damage that is being done to our planet and protect it for future generations.

The Disadvantages of Green Energy Resources
The benefits of green energy far outweigh and disadvantages but it is worth mentioning these here.
- It can be quite expensive to initially set up a green energy facility.
- We still have awhile to go before renewable energy can completely replace our current reliance on oil.
- There are powerful people who view green energy as a threat to their wealth and will put up barriers to its use.
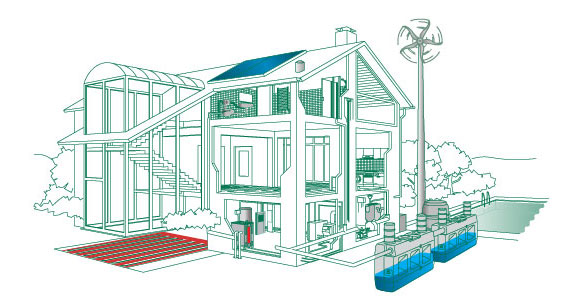
Home Renewable Energy
Most people are excited by the idea of home renewable energy. This would mean that we would be completely self-reliant and wouldn't have to worry about fuel bills arriving at our door. This is not just a dream and there are already people around the world who have managed to completely supply their own fuel needs - some even sell on the excess energy they create to the national grid.
It would be wonderful if all of us could manage this in the future. The technology is there and it is probably just a matter of time before it becomes an affordable option to convert to this type of system.
How to Use Renewable Energy?
At the moment there are a few different ways that we can use renewable energy. We could install solar panels or some of the other pieces of equipment that will help us create renewable energy. In the future we may all use renewable energy in different ways.
For some of us things might continue more or less as they do now with the only difference being that our energy supplier now uses a renewable resource to supply us. For other people the change will be much more dramatic in that we will take responsibility for supplying our home with renewable energy using equipment like wind turbines.

Why Choose Renewable Energy?
Choosing renewable energy just makes a lot of sense for us as individuals and for the planet as a whole. Our dependence on oil base fuels is leading to scarcity and as this happens the prices will continue to increase. Renewable energy will provide energy that will always be available and we should be less at the mercy of supply and demand. Renewable energy is also greener and this means that we are protecting the planet for future generations. Many people would claim that it is not so much about why we should choose, but more to do with the fact that we don't really have a choice.
New Green Energy Resources
It is the aim of cleangreenenergyzone.com to provide our readers with all the information they need about these exciting new green energy resources. We are letting them know that we have a choice and that there are gonig to be many advantages to choosing this alternative form of energy. A lot of people look upon green issues as being something that doesn't really affect them. We hope that by discussing the new green energy resources we can show how it can benefit people now. This is not about something vague and abstract but a change that can really make a difference to people's lives.

Homemade Energy Types and Benefits
There are a few different possibilities when it comes to homemade energy. One of the most popular options is the solar panel, but there are also other alternatives as well. Micro wind turbines are a good option but you may have to apply for planning permission and these can require quite a large initial investment - you will also need to make sure that you have good access to wind turbulence. The availability of your own energy source is something that is going to bring a lot of benefits to your life. Once you have made the initial investment you can then enjoy practically free energy; you should also find that such a system is not too expensive to maintain.
The Future of Alternative Energy
It seems certain that renewable energy is going to play an increasing part in our future lives. Most governments around the world seem to be actively promoting research in the area and have committed themselves to such a change. This is something that is not only desired but also necessary.
Most of us appreciate the fact that we can't rely on non-renewable energy forever so we need alternatives. We also know that our current consumption is damaging to the planet and we need to make a change. There is little doubt that our move towards alternative energy will continue with some of us leading the charge by creating our own homemade energy.
Renewable Energy Financing
Most governments around the world are keen to promote renewable energy and it is possible in many countries to receive assistance for this. What is available will very much depend on where you live but it can include such things as loans to improve your home energy system. Many governments also have funds which they use to encourage the move to greener power.
In order to discover if you are entitled to any type of financing it pays to do a bit of research; you may be surprised to find how much support there is available for this. (Green Energy Loans, Renewable energy credits and other funds)
There is also a lot of encouragement available for those who are willing to ensure that their homes are energy efficient; it is well known that many people waste energy through poor insulation and other sources of waste. The web is a great place to discover about what types of government funds are available in your area.